UnitedHealth Group is collaborating with Boston Scientific, Medtronic and the University of Minnesota to deploy 3,000 newly invented and U.S. Food and Drug Administration-authorized “light” ventilators. The emergency ventilator alternative devices will help address the limited stock of critical breathing support equipment in response to COVID-19.
Rapid Timeline to Meet High Demand
The collaboration went from concept stage to manufacturing in less than 30 days, with the first 500 products ready to ship this week. UnitedHealth Group is working with Medtronic and the University of Minnesota to determine initial destinations, while Boston Scientific is manufacturing and shipping the product.
These innovative devices will help address a clinical gap for patients who need a higher level of respiratory support but don’t have immediate access to traditional ventilators when there is a shortage. They will also provide health care workers with an additional tool to care for high volumes of patients who require emergency breathing support.
“We are grateful for collaborations with distinguished experts in academia, across health care and in other industries to offer innovative solutions like this light ventilator,” said Ken Ehlert, chief scientific officer, UnitedHealth Group. “Unique partnerships will be critical as we work together to confront COVID-19 in the months ahead. These light ventilators will help prevent future ventilation shortages, support patients who need this level of breathing assistance and expand clinical capacity to deliver care.”

From Concept to Completion
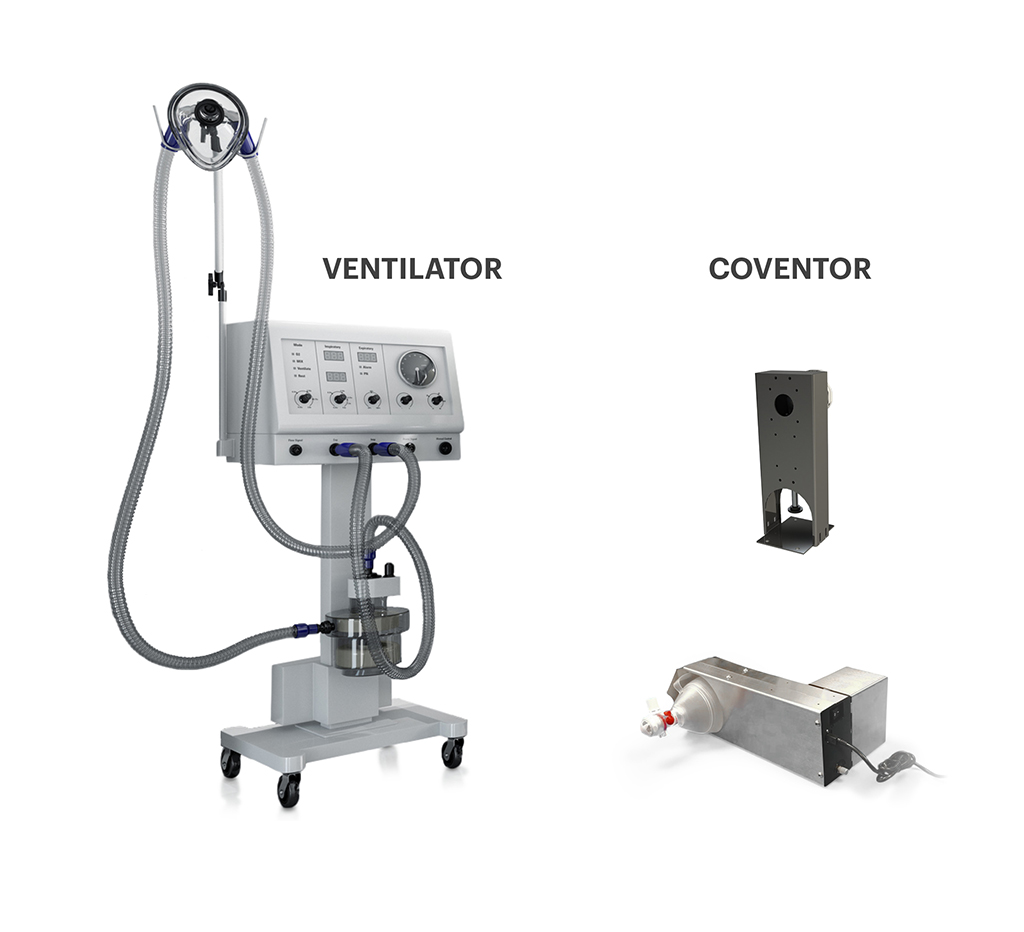
The University of Minnesota Medical School and Earl E. Bakken Medical Devices Center initially conceived the idea of the light ventilator, called the Coventor. Boston Scientific, Medtronic and UnitedHealth Group provided technical, clinical, regulatory and manufacturing expertise to refine how the device works and bring it to market at scale.
“We are appreciative of the support locally and nationally in getting the Coventor to the field,” said Stephen Richardson, M.D., cardiac anesthesiology fellow at the University of Minnesota Medical School. “We developed the Coventor to be useful in those clinical settings where traditional ventilators are not available. This allows patients who wouldn’t otherwise have the opportunity to survive, to survive. Making the emergency ventilator alternative devices as fast as possible, pushing them to people everywhere — that’s what this is all about.”
The emergency ventilator alternative device uses an electrically powered robotic arm to mechanically compress an off-the-shelf adult resuscitation bag, the sort often used by paramedics to help a patient breathe. This design provides oxygen assistance and enables health care workers to shift from manually operating the resuscitation bag. The device can also be configured with airflow accessories in multiple ways to best accommodate on-the-ground needs at different clinical facilities.
The devices will be shipped to geographies where ventilators are urgently needed, and any remaining devices will be offered as a donation to the U.S. Strategic National Stockpile.
Taking Bold Actions to Help Combat COVID-19
Collaborating to deploy the emergency ventilator alternative is the latest of several initiatives by UnitedHealth Group to combat COVID-19. Other initiatives announced to date include:
- Investing nearly $70 million to help at-risk populations and protect the health care workforce.
- Pioneering self-administered swab procedures to expand COVID-19 testing, reduce needed personal protective equipment and protect health care workers from unecessary exposure to COVID-19.
- Accelerating payments to providers throughout the crisis, with an initial tranche of nearly
$2 billion. - Waiving cost-sharing for COVID-19 testing and treatment for U.S. members of UnitedHealthcare plans and simplifying access to care by reducing prior-authorization requirements.
- Significantly expanding access to telehealth and virtual visits and redeploying 5,000 Optum clinicians to expand telehealth capabilities.
- Providing a special enrollment period for fully insured customers to allow employees who did not opt in for coverage during the regular enrollment period to secure coverage.
- Conducting proactive personal outreach to support seniors and the most vulnerable populations among our members.
- Launching a free nationwide emotional support line to manage the stress and anxiety caused by COVID-19.
- Converting company cafeterias to provide more than 75,000 meals a week for people in need and keeping our café team at work.

Share This Story